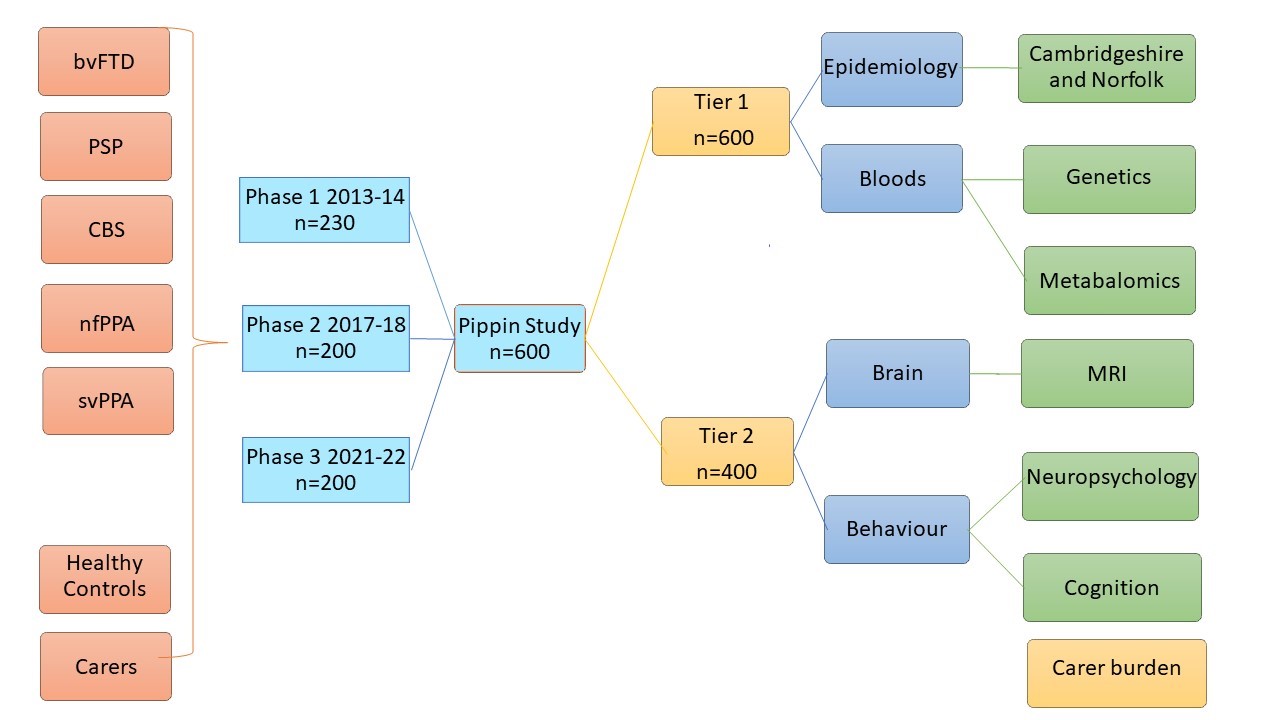
Pick’s disease and Progressive supranuclear palsy Prevalence and Incidence study (PiPPIN)
I am pleased to let you know that the PiPPIN study is starting active recruitment for its third phase (2021- 2022). We are looking to hear from ALL patients with below conditions and hope as many as possible will participate.
PiPPIN is an epidemiological study of disorders caused by frontotemporal lobar degeneration - including anyone in Cambridgeshire or Norfolk with:
- Progressive Supra-nuclear Palsy (PSP);
- Corticobasal Syndrome/Degeneration (CBD);
- Frontotemporal Dementia (FTD);
- Semantic Dementia;
- Primary Progressive Aphasia (PPA- semantic, non-fluent or logopenic variants)
PiPPIN ran between 2013-2014 and then 2017-18, and identified 365 people with one of these disorders, from multiple sources of referral, including:
- Neurology Department
- Hospital and community based psychiatry services
- Specialist nurses, for dementia and movement disorders
- Falls clinics
- Medicine for the elderly
- Patient based charities (PSP Association, FTD support group etc)
- Self-referral
Anybody with a relevant diagnosis can take part – the simplest level of participation is to agree to basic collection of demographic and diagnostic data (Tier 1). There is an optional blood test at this stage. If you are willing, the next level of participation includes neuropsychological tests and an MRI brain scan.
We are also looking to recruit around 50 healthy controls – between the ages of 50-80 to participate in Tier 1 and 2.
There are 3 stages for the study.
- Step 1. To collect epidemiological data for all patients with a Fronto-temporal degenerative condition who present between January 2021 and December 2022 in Cambridgeshire, Peterbourough and Norfolk. This includes age, sex, ethnicity and diagnosis of ALL patients presenting with a relevant disease.
- Step 2. To give permission for a few more personal details such as years in education, job, other health problems and to consider a blood test.
- Step 3 To participate in active research such as Neuropsychology tests, an MRI scan and Carer’s questionnaires.
- Most of the testing will occur at the Cambridge Centre for Parkinson Plus at the Addenbrookes Hospital site.
- Our team can arrange community assessments, for those who would find it difficult to travel. For particularly fragile participants travelling from afar, we are able to provide overnight accommodation.
For patients: Please contact us on kcg30@medschl.cam.ac.uk or by telephone (01223764674) if you would like an information pack or to find out more. We can arrange community assessments, for those who would find it difficult to travel. Informed consent to participate would follow, after written information is sent and you get an opportunity for questions to the study team.
For clinicians : We are not asking you to consent people to take part in the study. Rather, to refer them to the study team, or to provide them with information about the study. Verbal consent is sufficient for referral to the study team, with contact details (either Addenbrookes to Addenbrookes email to katherine.stockton@addenbrookes.nhs.uk or securely via nhs.net to katherinestockton@nhs.net). The patient's decision to participate in Tier 2 and informed consent would follow, after written information and an opportunity for questions to the study team.
Anybody with a relevant diagnosis can take part – the simplest level of participation is to agree to basic collection of demographic and diagnostic data. There is an optional blood test at this stage. If the patients are willing, the next level of participation includes neuropsychology and an MRI brain scan.
For more information, or to refer a potential participant, please contact us via (nhs) email, or phone:
Katherine Stockton
Neurology Clinical Research Registrar to Professor James Rowe, University of Cambridge.
01223764674. This number is not manned daily so please leave a message and we will return your call.
katherine.stockton@addenbrookes.nhs.net